
Background and COntext
The FDA's Small Business Determination (SBD) program allows eligible businesses to qualify for reduced user fees before submitting a regulatory application. The original digital experience (SBD V1) required users to download, fill out, and upload PDFs containing business information, which is a process prone to errors, omissions, and inconsistencies. As part of the FDA's broader Digital Transformation (DT) program, SBD was selected for improvement. I took on the UX lead role as SBD V1 was being implemented and V2 planning began. My focus was to evolve the experience from a static upload form to a more dynamic, guided web form aligned with enterprise standards and internal FDA review processes.

.gif)
Problem Space
SBD V1 had several key challenges:
- Limited validations, allowing for incomplete or inaccurate submissions.
- A heavy reliance on PDF uploads for critical data
- No visibility into what happens after the user clicks "submit"
- UI inconsistencies that made the experience difficult to follow
This raised broader questions: How is this data used internally? How does it reach reviewers? And how can we improve both the applicant's and the agency's experience?
My role
I led UX efforts for SBD V2, working across discovery, audit, design, and Agile implementation. I collaborated with product owners, developers, and policy stakeholders to:
- Audit the current UI and identify inconsistencies
- Design key improvements to the webform
- Define validation logic and dynamic behaviors
- Explore how submitted data is handled internally
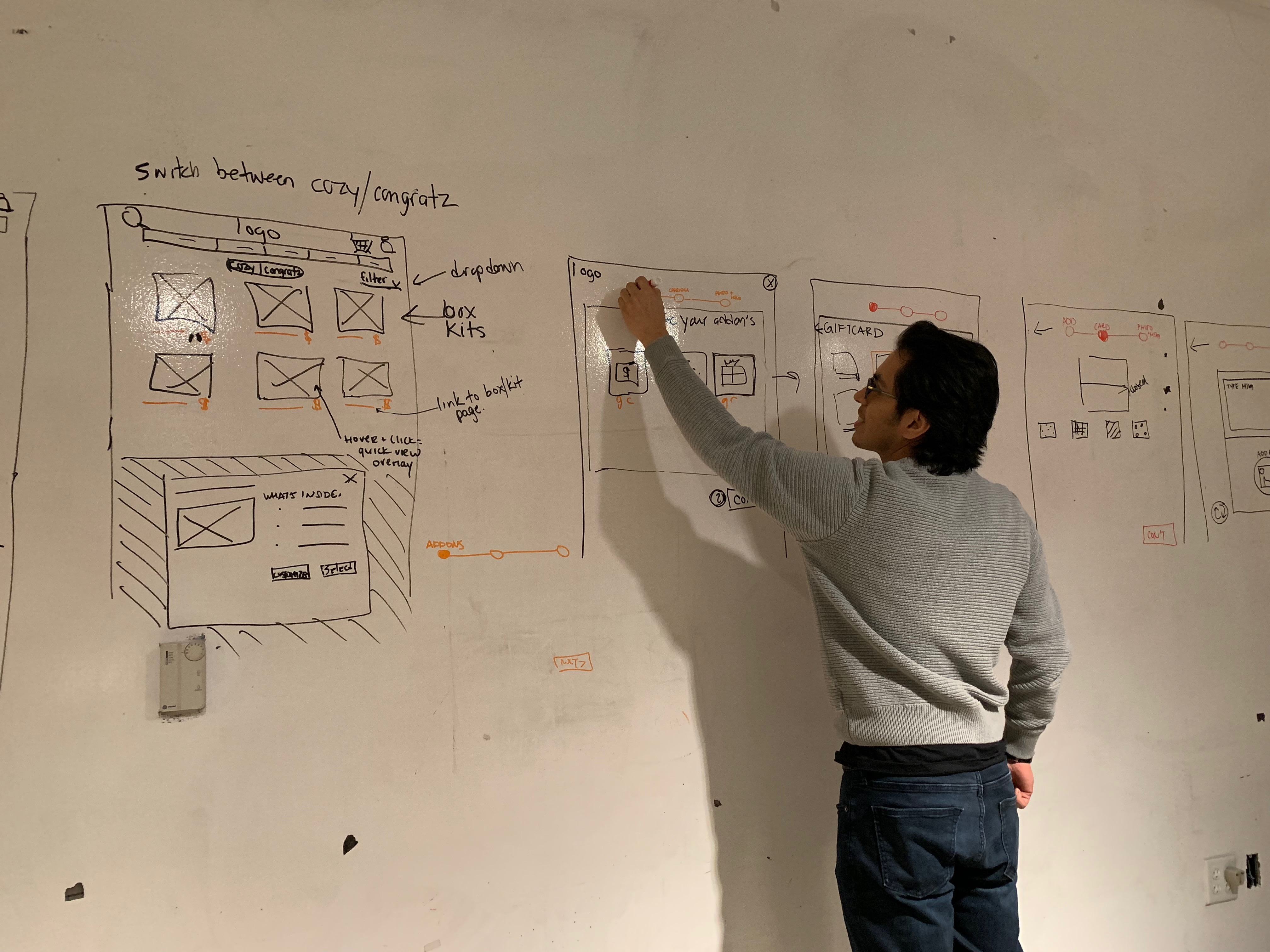
Key Contributions
Expanding the Lens: Mapping the DCC Process
To better understand how submission design impacts internal processing, I mapped the end-to-end DCC workflow, including:
- Submission intake (paper or electronic)
- System assignment (e.g., Century)
- Verification and transcription (manual entry or loader tool)
- Quality checks and finalization for reviewer readiness
This journey map helped identify key friction points and manual steps that could be improved if upstream submission forms (like SBD) were designed with those needs in mind.

Insights from DCC Journey Mapping
- Manual Transcription Bottlenecks: Many submissions required rekeying of information into internal systems, which delayed processing.
- File Format Issues: Format mismatches or incorrect uploads from industry users resulted in back-and-forth corrections.
- Lack of Feedback Loops: Submitters were unaware of issues until long after submission, increasing frustration and support needs.
- Missed Opportunities for Automation: With better structured data (like from SBD V2), auto-ingest into transcription tools could reduce workload and errors.

Cross Team Facilitation & Impact
I facilitated cross-functional discussions between the SBD, DCC team and internal tools team ( CTS & CEntry). These conversations suirfaced the opportunity for auto -ingestion ( automatically transferring structered data from webform into internal trasncription tools.
By advocating for this connection between frontend experience and backend review wqorkflows, I helped shift the conversation from form usability to submisison lifecycle efficiency.